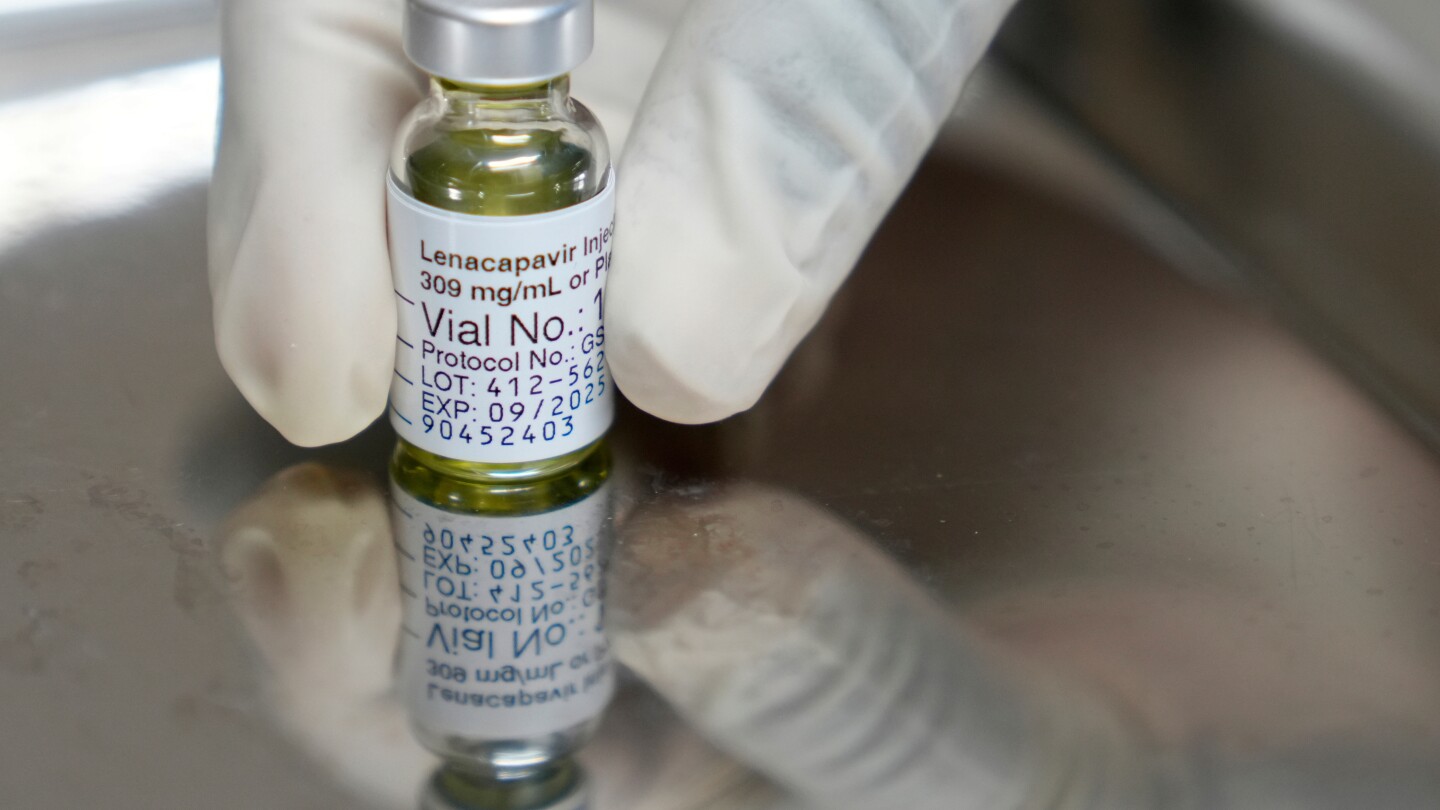
Eswatini has made history by becoming the first African nation to introduce lenacapavir, a revolutionary twice-yearly HIV prevention injection. Developed by Gilead Sciences, this groundbreaking drug has shown near-total protection in clinical trials and is hailed as a transformative tool in combating HIV, a virus that has claimed millions of lives across the continent. The rollout is part of the U.S. President’s Emergency Plan for AIDS Relief (PEPFAR), in collaboration with the Global Fund, aiming to benefit at least 2 million people in 10 high-risk African countries by 2027. Eswatini, with the world’s highest HIV incidence, received the drug in the same year as its U.S. approval, marking a significant milestone. Zambia also received its first shipment, while regulatory approvals are pending in Botswana, Kenya, Malawi, Namibia, Rwanda, Tanzania, Uganda, and Zimbabwe. The U.S. initially planned to distribute 250,000 doses in 2024 but increased this to 325,000 due to high demand. In Eswatini, approximately 6,000 high-risk individuals, particularly mothers and newborns, will benefit from the initial rollout. Despite its potential, concerns over limited supplies and manufacturing rights have sparked debates, with South Africa’s health minister highlighting the need for broader access. Gilead has significantly reduced the drug’s price for lower-income countries, making it more accessible. However, civil society groups in South Africa have criticized the exclusion of local manufacturers from licensing agreements, despite their contributions to clinical trials.