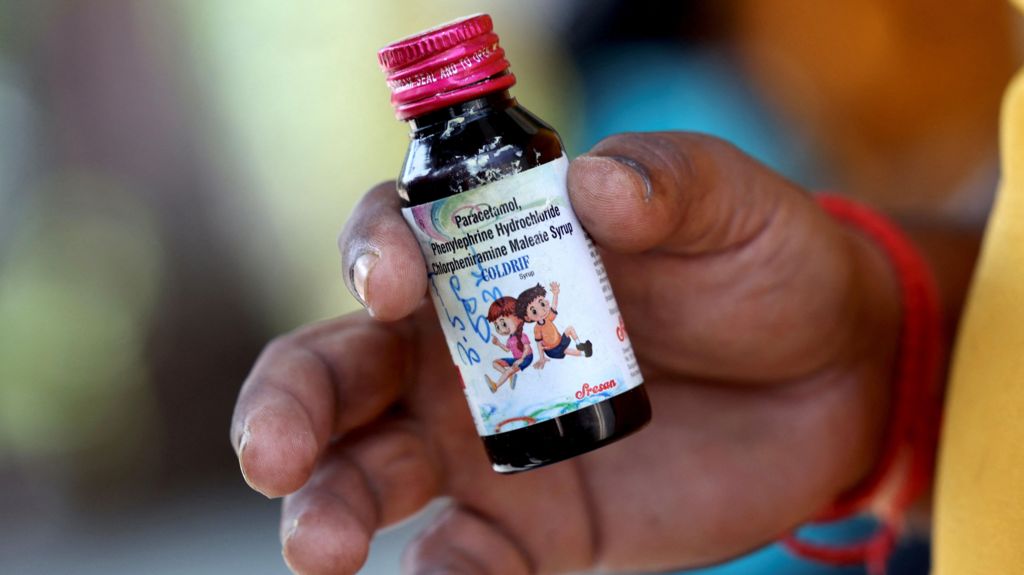
The World Health Organization (WHO) has expressed profound concern regarding lapses in India’s drug safety regulations following the tragic deaths of at least 20 children linked to contaminated cough syrups. The fatalities, reported in Madhya Pradesh and Rajasthan over the past month, have been traced to three specific syrups—Coldrif, Respifresh, and ReLife—found to contain diethylene glycol (DEG), a toxic chemical commonly used in industrial solvents. The Indian government has responded by arresting the owner of Sresan Pharmaceuticals, the manufacturer of Coldrif, halting production, and initiating a comprehensive investigation. The WHO has also warned that these tainted medicines could potentially reach other countries through unregulated distribution channels. In Tamil Nadu, health authorities have permanently revoked the manufacturing license of Sresan Pharmaceuticals after an inspection revealed 364 violations, including unhygienic storage conditions, substandard water quality, and lack of quality assurance protocols. The incident has sparked national outrage and raised concerns among parents, as oral syrups are commonly administered to children in India. This is not the first time Indian-made cough syrups have come under scrutiny; similar cases in The Gambia and Uzbekistan in 2023 resulted in the deaths of 88 children. The recurring issue highlights systemic failures in India’s pharmaceutical regulatory framework and underscores the urgent need for stricter oversight and accountability.