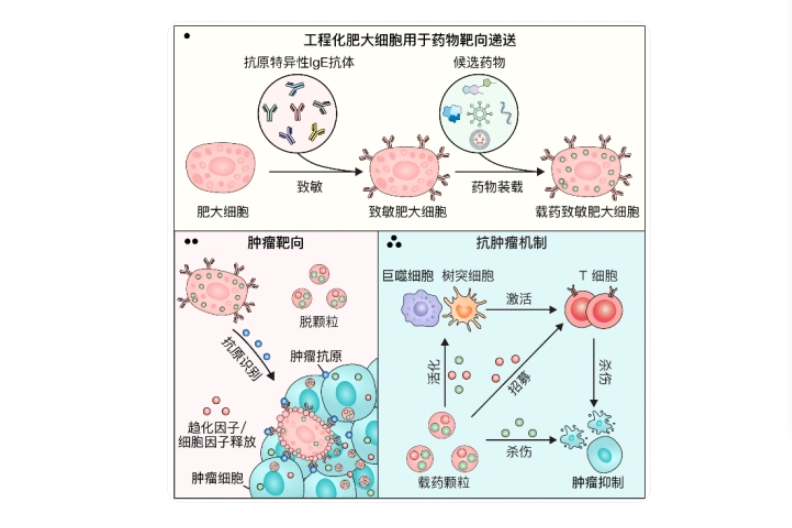
In a groundbreaking development published in the journal Cell, Chinese researchers have successfully engineered one of the body’s most rapid immune mechanisms into a potent weapon against cancer. The innovative approach leverages mast cells—typically associated with allergic reactions like sneezing and hives—to trigger targeted inflammatory attacks within tumors.
The collaborative research effort between Zhejiang University and the First Hospital of China Medical University focused on reprogramming these immune cells to recognize and assault cancer tissue. Professor Gu Zhen from Zhejiang University’s School of Pharmacy explained that the team drew inspiration from the extreme responsiveness of allergic reactions to overcome tumor immunosuppression.
Through sophisticated bioengineering, scientists equipped mast cells with tumor-specific IgE antibodies that function as precision guidance systems. When administered intravenously, these modified cells migrate directly to cancerous growths and discharge their inflammatory payload upon contact with target cells.
This deliberately induced allergy-like reaction within the tumor microenvironment transforms immunologically ‘cold’ tumors—those typically invisible to immune detection—into ‘hot’ tumors that become vulnerable to attack by cancer-killing T-cells. The strategy demonstrated significant efficacy across multiple mouse models including melanoma, breast cancer, and lung metastases.
The research team further enhanced the platform by utilizing mast cells as biological delivery vehicles for oncolytic viruses. By concealing these tumor-destroying viruses within protective cellular vesicles, the system ensures safe transport through the bloodstream until activation at the cancer site.
Notably, the technology proved effective in human-derived tumor models using mast cells targeted against the HER2 cancer marker. This success indicates potential for personalized cancer treatment through matching IgE antibodies to patient-specific tumor markers.
Beyond viral delivery, the mast cell platform demonstrates versatility in transporting diverse therapeutic agents including conventional drugs, proteins, antibodies, and nanomedicines. Researchers envision a future where multiple treatment modalities could be integrated within a single cellular therapy system.
The research team is now developing workflows for patient-specific antibody selection, scaling manufacturing processes for therapeutic mast cells, and exploring combinations with existing immunotherapies to accelerate clinical application.